Abstract
Introduction: hematological malignancies (HM) are frequently diagnosed in elderly patients but chronological age is inaccurate and unable to predict the real functional reserve of patients. For this reason, comprehensive geriatric assessment (CGA) is the gold standard procedure, not only to assess frailty but also to predict treatment toxicity and survival. It can also help to tailor specific treatment to frailty status and to implement geriatric interventions. The aim of this study is to analyze the impact of CGA in patients with HM in a real-life setting.
Methods: all patients referred for CGA between February 2019 and December 2021 were included in this single-center analysis. Patients were referred by their hematologist based on clinical judgement. CGA was performed by a specialized geriatrician who also made recommendations on treatment intensity, implemented interventions and subsequent follow up for patients in need. Geriatric domains were assessed using the following scales: G8 (screening), Charlson (comorbidities), Barthel (activities of daily living (ADL)), Lawton (instrumental ADL (IADL)), SPPB and gait speed (physical function), Fried (frailty), mini-mental (cognition), GDS-SF (mood), MNA-SF (nutrition), polypharmacy (≥5 medications). SPSS software version 21.0 was used for statistical analysis.
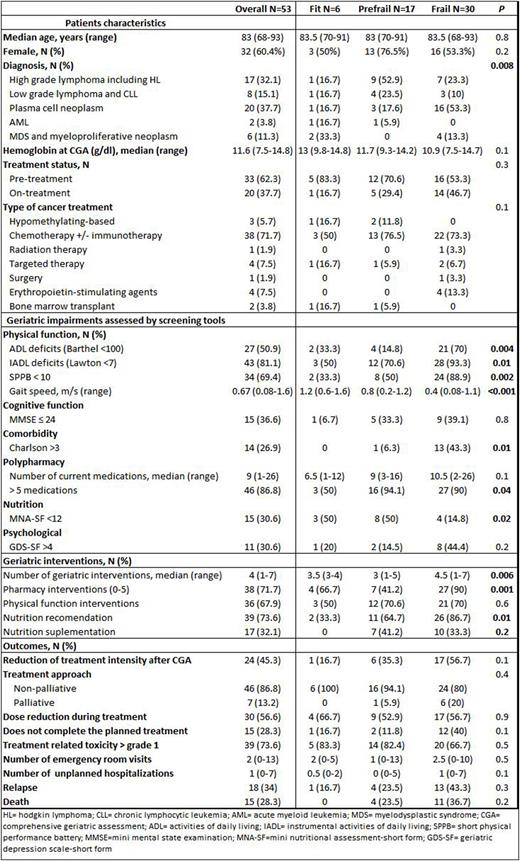
Results: Sixty-nine consecutive patients were referred for CGA. Of these, 53 with HM requiring or undergoing specific treatment were analyzed. The other 16 were excluded because of death before CGA, loss of follow up or non-oncologic diagnosis. Median age was 83 years-old (68-93) and all patients had a G8 score < 14. Frailty according to Fried´s criteria divided patients into 3 groups: fit 11.3%; pre-frail 32.1% and frail 56.6%. Patient's characteristics, geriatric impairments and interventions, as well as the clinical evolution of the entire cohort and of the patients according to frailty status are summarized in table 1. Lymphomas and plasma cell neoplasms were the most frequent diagnoses (84.9%) and were mainly treated with immunotherapy +/- chemotherapy-based regimens (71.7%). Frailty was significantly associated with physical function, comorbidity, nutrition and polypharmacy. A high proportion of patients (96%) required at least one geriatric intervention. The number of interventions and the proportion of patients receiving them was significantly associated to frailty status in all domains except physical function (table 1). Dose intensity was reduced after CGA in almost half of the patients (45.3%), which had no significant impact on progression (p=0.2). We found that ADL dependencies and cognitive function were able to predict unexpected hospitalizations (UH) (OR 4.08 [95% CI = 1.24-13.43] and 4.67 [95% CI = 1.06-20.53]) while ADL dependencies and mood (OR 5.00 [95% CI = 1.18-21.04] and OR 4.80 [95% CI = 1.03-22.37]) predicted emergency room visits. With a median follow-up time since CGA of 19 months (2-40), median OS was not reached, with an estimated 2-year OS rate of 72%. Impairment of ADL (p=0.02) and comorbidities (p=0.04) were significantly associated with OS. Other factors with impact on OS were male gender (p=0.01), palliative approach (p=0.02), toxicity (p=0.01) or UH (p=0.01). Multivariate analysis showed that male sex (p=0.004) and UH (p=0.04) remained independent factors associated with OS.
Conclusions: CGA, with impact in the oncological decision-making process and specific interventions, is a feasible and beneficial real-life practice, as frailty is common in older patients with HM. Functional impairments and comorbidities were predictors of OS, despite the heterogeneity of this cohort with respect to the type of underlying diseases and treatments administered. Impairments in ADL, mood and cognitive function were able to predict unexpected utilization of health-care services. Geriatric interventions are warranted to try to reverse frailty in this population.
Disclosures
González-gascón-y-Marín:Janssen, Abbvie, Astrazeneca, Sanofi, Amgen: Honoraria, Speakers Bureau. Landete:Janssen: Speakers Bureau. Hernández-Rivas:Janssen, Roche, Abbvie, AstraZeneca, Beigene, Lilly, Gilead, BMS-Celgene, Amgen, Takeda, Jazz Pharmaceuticals, Rovi, Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen, Roche, Abbvie, AstraZeneca, Gilead, BMS-Celgene, Amgen, Takeda, Astra Zeneca: Speakers Bureau; BMS-Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal